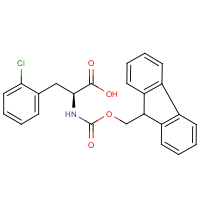
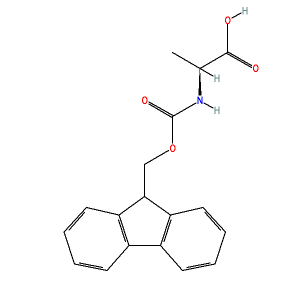
Arylboronate Ester Protected Amino Acids as Orthogonal Building Blocks for Fmoc Solid‐Phase Peptide Synthesis - Liu - 2017 - European Journal of Organic Chemistry - Wiley Online Library

Development of Fmoc-Protected Bis-Amino Acids toward Automated Synthesis of Highly Functionalized Spiroligomers | Organic Letters

Synthesis of Fmoc-protected 4-N,N,-dimethylaminophthalimidoalanine (1)... | Download Scientific Diagram

An Fmoc protecting group can be removed from an amino acid by treatment with the amine base piperidine. Propose a mechanism. | Homework.Study.com

Carpino's protecting groups, beyond the Boc and the Fmoc - El‐Faham - 2020 - Peptide Science - Wiley Online Library
Fmoc-L-Lysine-(Boc), 5 g, CAS No. 71989-26-9 | Fluorenylmethylene / Fmoc | Amino acids, protected | Amino Acid Derivatives | Amino Acids and Amino Acid Derivatives | Organic & Bioorganic Chemicals | Chemicals | Carl Roth - International

Fmoc-OASUD: A new reagent for the preparation of Fmoc-amino acids free from impurities resulting from Lossen rearrangement - ScienceDirect

An efficient and expeditious Fmoc protection of amines and amino acids in aqueous media - Green Chemistry (RSC Publishing)

Prevention of aspartimide formation during peptide synthesis using cyanosulfurylides as carboxylic acid-protecting groups | Nature Communications